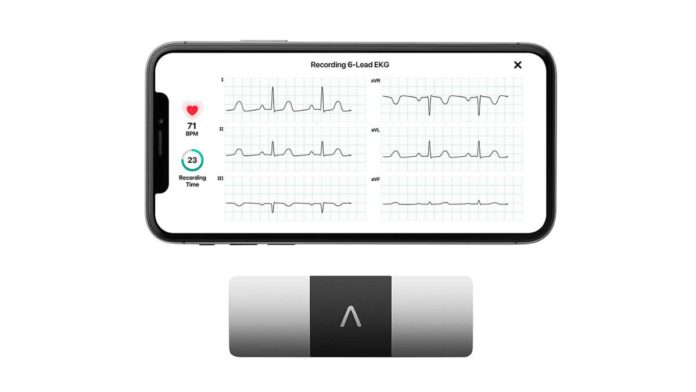
Health Minister Dr. Sapam Ranjan has revealed a new FDA-approved ECG device, called KardiaMobile 6L, AI and Bluetooth enabled and approved by FDA. the device was revealed at Classic Grande hotel in Imphal.
Dr. Ranjan explained that many people suffer from cardiovascular diseases, and the number has increased in the region. He added that there had been situations where patients could not figure out what was wrong and died even before medical assistance could be provided.
To solve this problem, the state government took an initiative to enhance home screening of non-communicable diseases (NCD) and develop such devices under the CMHA scheme. Dr.Ranjan also said that there is a need to incorporate technology and artificial intelligence to help monitor patients away from medical help.
Read More: German BioNTech To Acquire British AI Startup InstaDeep
This is why, AliveCor developed the KardiaMobile 6L ECG device, an AI startup developing machine-learning algorithms for ECG sensors. It can be linked to a mobile phone and provides results within 30 seconds.
Munish Yadav, AliveCor’s sales and marketing director, facilitated the launch along with state mission director NHM Dr. Somorjit Ningombam.